Technical Summary
Introduction
 Electronic waste (e-waste) refers to discarded electronic devices, such as computers, smartphones, and household appliances, at the end of their useful life.
Electronic waste (e-waste) refers to discarded electronic devices, such as computers, smartphones, and household appliances, at the end of their useful life.
With the rapid advancement of technology and growing demand for electronic products, e-waste has become the fastest-growing waste stream worldwide.
In 2022, 62 billion kilograms of e-waste was generated, a sharp increase from previous years. However, despite this vast amount, only 22% of e-waste was formally recycled, with much of it ending up in developing countries where it is processed under unsafe conditions.
In some regions, open burning methods are used to extract metals from e-waste, releasing toxic chemicals into the air, soil, and water, which pose significant health risks to local communities.
Additionally, many have old/unused electronic devices at home that could be recycled, but due to privacy concerns they are not.
Tin in demand
 Tin is an essential metal, widely used in modern electronics for soldering components in devices such as smartphones, computers, and circuit boards. Its corrosion resistance and excellent conductivity make it irreplaceable in the technology sector.
Tin is an essential metal, widely used in modern electronics for soldering components in devices such as smartphones, computers, and circuit boards. Its corrosion resistance and excellent conductivity make it irreplaceable in the technology sector.
Beyond electronics, tin plays a vital role in renewable energy technologies like solar panels and electric vehicles.
The increasing demand for tin in both established and emerging technologies highlights the need for a reliable and stable supply to support ongoing and future industries.
Printed circuit boards (PCBs) contain on average 3% tin. However so far e-waste recycling has been focused on the recovery of precious metals, copper and other elements.
While primary mining remains a significant source of tin, recycling e-waste offers an important route to increase sustainable future tin supply.
Global e-waste generation and recycling trends
 The latest data from the Global E-Waste Monitor 2024 reveals significant trends in e-waste generation and recycling, highlighting both the challenges and opportunities for managing this ever-expanding waste stream.
The latest data from the Global E-Waste Monitor 2024 reveals significant trends in e-waste generation and recycling, highlighting both the challenges and opportunities for managing this ever-expanding waste stream.
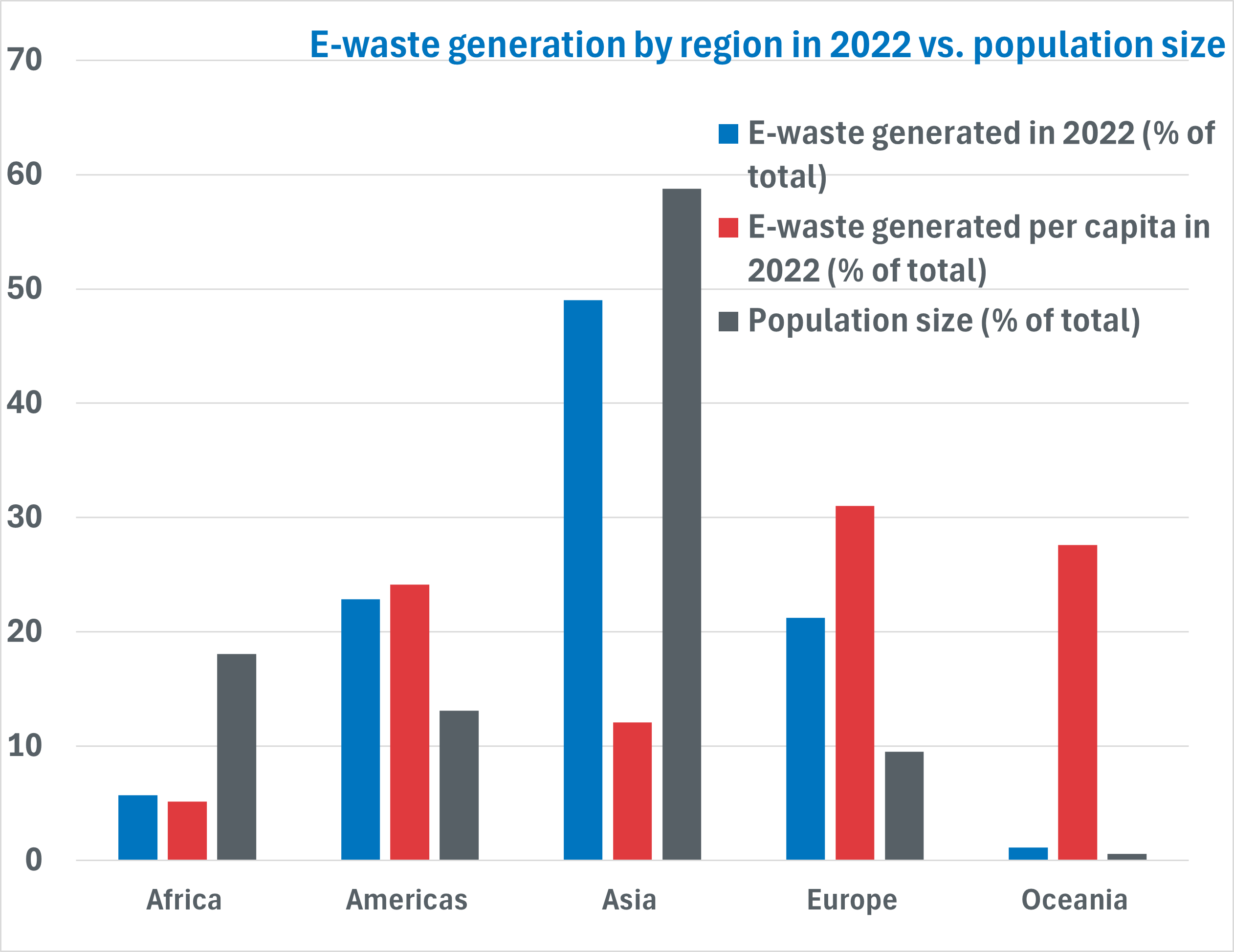
The graph illustrates the distribution of e-waste generation by region in 2022, with Asia being the largest contributor, generating 30 billion kg (48%) of the global total. This is followed by Europe (14 billion kg, 23%), Americas (13 billion kg, 21%), and Africa (3.5 billion kg, 6%).
However when looking at e-waste generation per capita, Europe leads with 18 kg per person, followed by Oceania at 16 kg per person, and the Americas at 14 kg per person. Asia and Africa have significantly lower per capita figures, with 7 kg and 3 kg, respectively.
If we compare this to population size, it is clear that Asia has the highest e-waste generation due to it having such a large population. However, when we look at per capita rates, most members of the population are using less electronics than other regions. For example, Europe has a lower population, but a high per capita e-waste generation.
Oceania on the other hand has the lowest e-waste generation overall due to its small population. Oceania’s per capita generation however is high, showing that the majority of the population is using more electronics on average than the Asian population for example.
Africa has a large population, but its e-waste generation overall and per capita is low.
This demonstrates that, while total e-waste generation is highest in Asia due to its large population, regions like Europe, Oceania and the Americas produce more e-waste on a per-person basis, reflecting the high demand for electronics in these regions.
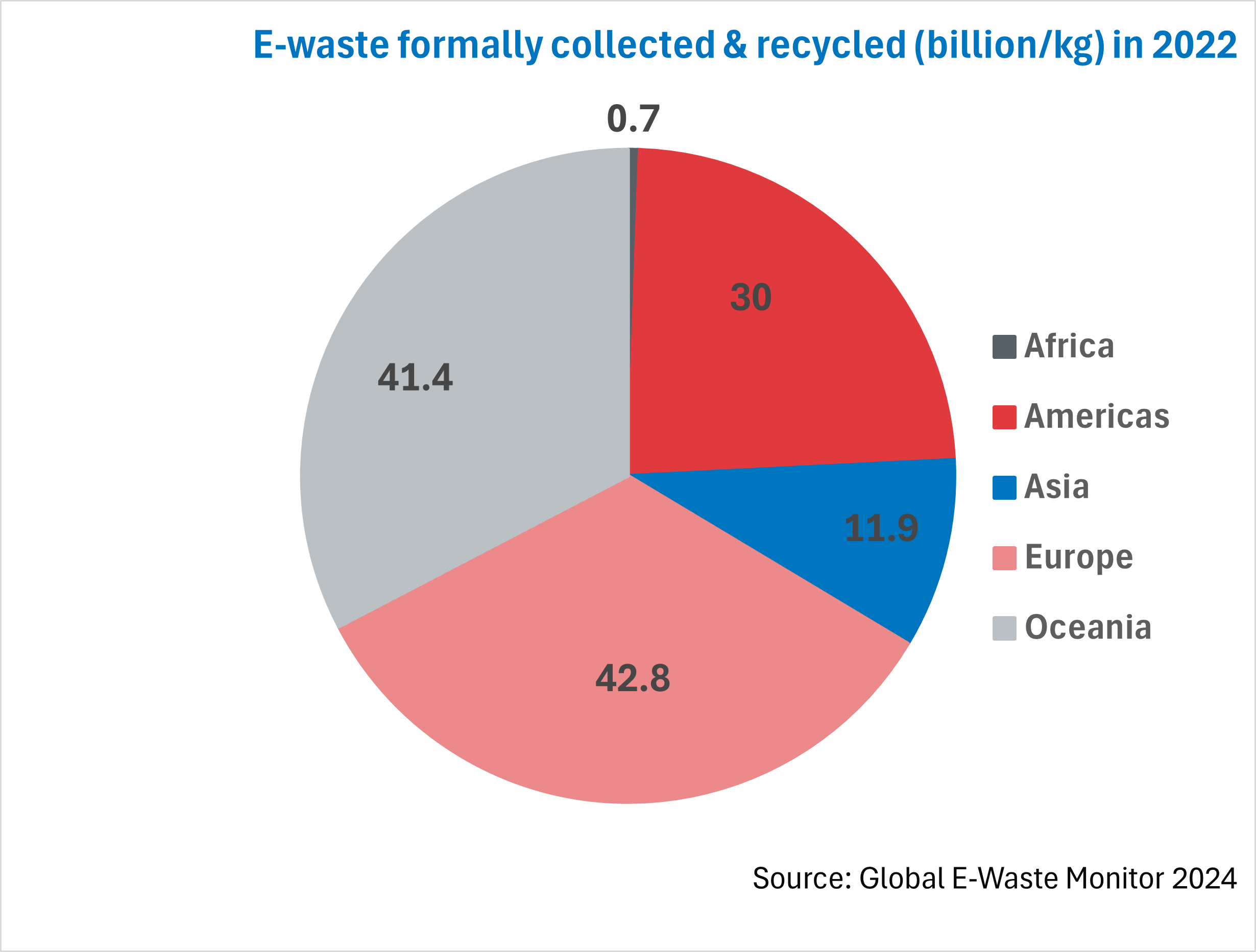 The chart illustrates the amount of e-waste that was formally collected and recycled out of the e-waste generated in each region in 2022.
The chart illustrates the amount of e-waste that was formally collected and recycled out of the e-waste generated in each region in 2022.
Europe again leads the way with 5.6 billion kg (42.8%) of e-waste recycled. This is thanks to legislation in place such as the extended producer responsibility (EPR) principle. The EPR principle encourages manufacturers to design eco-friendly products as they are responsible for their products even at end-of-life. 37 European countries adhere to the EPR principle.
Following closely behind is Oceania with 41.4% of e-waste recycled in 2022. This is due to e-waste recycling giants Oceania, such as Sircel in Australia, having the infrastructure in place to recycle large volumes of e-waste.
The Americas recycled 30% of e-waste in 2022 thanks to recycling infrastructure, legislation such as the EPR principle and e-waste collection targets.
Asia follows with almost 12% recycled with 11 countries adhering to the EPR principle and 18 countries having national policies, legislation or regulations in place.
Africa falls behind regarding the volume of e-waste collected and recycled. This can be due to a lack of recycling infrastructure in Africa, as well as the lack of legislation in the continent.
The main e-waste component: PCBs
 PCBs are key components in most electronic devices, connecting various parts like resistors, capacitors, and circuits. PCBs usually contain around 30-50% metal, and 50-70% non-metal components.
PCBs are key components in most electronic devices, connecting various parts like resistors, capacitors, and circuits. PCBs usually contain around 30-50% metal, and 50-70% non-metal components.
PCBs contain tin as electronic solder (3% tin on average). They also contain valuable metals like gold, silver, and copper, along with hazardous materials such as lead and flame retardants.
Proper recycling of PCBs is essential to recover these metals and minimise environmental impact. Specialised methods are needed to effectively extract valuable materials while reducing harm to the environment.
Pre-processing
Pre-processing is a critical phase in the recycling of electronic waste (e-waste), as it prepares the materials for the efficient recovery of valuable metals, while minimising harmful environmental effects. This phase involves several stages, each designed to separate, reduce, and isolate different materials present in e-waste.
Collection and sorting
Once collected, e-waste is categorised based on type, size, and material content. This sorting process ensures that different types of electronics are handled separately. For example, devices like mobile phones contain a higher concentration of precious metals, while larger electronics like televisions and washing machines contain more glass and plastic.
Dismantling
Dismantling is a pivotal step in the e-waste recycling process, particularly for the effective extraction of valuable materials from PCBs, which contain essential metals such as tin. To ensure efficient recovery of these materials, a variety of methods are employed, ranging from manual to advanced robotic techniques. In some cases, dismantling also involves chemical and thermal depopulation methods, designed to further facilitate the removal and recovery of valuable metals.
Manual and Robotic Dismantling
Manual dismantling remains a widely used approach, especially when working with small batches of e-waste. In this process, workers carefully disassemble electronic devices, removing PCBs from their casings, batteries, and other components. While labor-intensive, this method allows for the selective separation of PCBs, which can then be processed to recover valuable metals.
For larger-scale operations, robotic dismantling has become increasingly popular. These systems use automated machinery to remove PCBs from electronic devices with greater speed and precision. Robotic systems can be programmed to handle a variety of devices, ensuring the efficient extraction of PCBs while minimising human error and exposure to harmful substances. In some systems, robotic arms equipped with specific tools can disassemble complex devices like computers, televisions, and mobile phones, ensuring that components are removed without causing damage.
Chemical and Thermal Depopulation
Chemical and thermal depopulation methods are increasingly used to simplify the dismantling process, especially when recovering tin from e-waste.
-
Thermal Depopulation: This method involves heating the e-waste to melt the soldering materials that hold the components to the PCBs. Tin-based solders are amenable to this process as they have relatively low melting points. By applying controlled heat, solder joints can be melted, allowing for the easy removal of components from the PCB. External actions such as shaking the board can assist with the removal of components. This process not only facilitates the dismantling of components but also helps in recovering tin from the soldering material itself. Advanced thermal techniques can be combined with pyrometallurgical processes to further recover and purify tin and other valuable metals.
-
Chemical Depopulation: Chemical depopulation involves using solvents or acids to dissolve solder and remove components from PCBs. For tin recovery, commonly used acids include hydrochloric acid (HCl), often combined with oxidants such as hydrogen peroxide (H₂O₂) or ferric chloride (Fe³⁺) to enhance tin dissolution. The acid breaks down the tin in solder joints, leaving behind valuable metals, which can be separated in subsequent processing steps. Chemical methods can be further optimised by employing selective reagents that target specific materials, thus improving the recovery rate of tin while minimising the loss of other precious metals. A project facilitated by the International Tin Research Institute (ITRI, now ITA) and Ultromex (ITRIMEX project) explored the use of ferric chloride (FeCl3) with nitric acid alongside ultrasonic agitation to successfully recovery tin from PCBs. Adjustment of the solution to pH 4 enabled precipitation of tin that could be recovered via electrowinning.
X-ray sorting and advanced material identification
Once e-waste is sorted by type, X-ray sorting technology is used as one of the first advanced sorting methods in the pre-processing stage. X-ray fluorescence (XRF) helps identify and separate materials based on their elemental composition. In this method, high-energy X-rays are emitted to excite the atoms in the material, causing them to emit secondary X-rays that are characteristic of specific elements.
In e-waste recycling, XRF is particularly effective for:
- Identifying and quantifying metals like tin, gold, silver, copper, and other valuable elements, even in trace amounts.
- Detecting hazardous substances like lead, mercury, or cadmium that need to be handled separately during recycling.
- Optimising material sorting by allowing automated systems to segregate materials based on metal content, ensuring that valuable metals like tin are efficiently recovered from PCBs and other components.
Using X-ray sorting early in the pre-processing chain improves sorting accuracy and increases the efficiency of downstream recovery processes by isolating valuable materials before other mechanical processes are applied. The SCARCE Alliance for example between CEA in France and Nanyang Technological University in Singapore have designed a convolutional neural network-based optical recognition with XRF with 96.9% accuracy for advanced e-waste sorting.
Mechanical shredding
Dismantled e-waste is sent through mechanical shredders, which break down the materials into smaller pieces. This shredding process increases the surface area of the materials, making it easier to separate them in subsequent stages. The mechanical action also helps break apart composite materials, such as plastics embedded with metals.
Magnetic separation
Once shredded, magnetic separation can be used to isolate ferrous metals (such as iron and steel) from the non-ferrous materials like copper, tin, and aluminium. Magnets attract ferrous materials, leaving the non-ferrous metals behind.
Eddy current separation
Eddy current separation is employed to separate non-ferrous metals based on their conductivity. In this process, an eddy current separator uses a rotating drum with a magnetic field to induce currents in non-ferrous metals, creating a repulsive force that ejects them from the material stream. This method is particularly useful for separating:
- Aluminium, which is light and non-magnetic but still highly conductive.
- Copper, tin, and other non-ferrous metals, which are separated from other non-metallic materials like plastics and glass.
Density separation
 Water-based separation: This technique involves placing the e-waste in water. Lighter materials (such as plastics) float to the top, while heavier metals sink to the bottom. Other mediums can be used other than water, including sand, similarly to geological processing.
Water-based separation: This technique involves placing the e-waste in water. Lighter materials (such as plastics) float to the top, while heavier metals sink to the bottom. Other mediums can be used other than water, including sand, similarly to geological processing.
Air-based separation: In this method, an air stream is used to separate materials. Lighter items are lifted by the air, while heavier materials fall due to their greater density. This method is often used in combination with shredding to sort the materials into fine fractions, making it easier to isolate valuable metals for further recovery.
After pre-processing further techniques are employed to isolate key materials. An example of a company employing density separation is EnviroLeach. EnviroLeach combines mining and crushing technologies with density based separation to produce a metal concentrate (see picture) for further processing.
Metals recovery routes
Hydrometallurgical
Hydrometallurgical techniques use aqueous solutions such as acids to extract metals from e-waste. Hydrometallurgical recovery of tin from e-waste typically involves several core steps:
- Leaching the tin into solution, separating/purifying the tin-laden solution (often via solvent extraction or ion exchange)
- Recovering tin in metallic or compound form (via electrowinning, chemical reduction, or precipitation), and managing any secondary residues.
Throughout these steps, the goal is to maximise tin yield and purity while minimising co-dissolution of unwanted elements.
Acidic Leaching
 Acid leaching is a direct way to dissolve tin from solder present in e-waste. The choice of acid and oxidising conditions is critical because tin can exist in multiple oxidation states (0, +2, +4) and form insoluble species.
Acid leaching is a direct way to dissolve tin from solder present in e-waste. The choice of acid and oxidising conditions is critical because tin can exist in multiple oxidation states (0, +2, +4) and form insoluble species.
Simple acids alone often struggle with tin. Tin requires an oxidant to form soluble ions – either Sn²⁺ or Sn⁴⁺ coupled with complexing anions. Hydrochloric acid (HCl) is a popular choice for tin leaching because chloride anions can form complex ions like SnCl₆²⁻, which help keep Sn⁴⁺ in solution.
However, pure HCl may not oxidise tin sufficiently on its own. Researchers have improved HCl leaching by adding oxidants or additional chloride sources. A recent study showed that 6 M HCl alone leached about 78% of tin from pulverised PCB dust, but adding 3 M NaCl (to enrich chloride content and possibly catalyse oxidation) boosted tin leaching to 94.8%2.
Other oxidants like hydrogen peroxide (H₂O₂) or ferric chloride (Fe³⁺) are also documented to enhance tin dissolution by chemically oxidising Sn(0) to Sn(II/IV). Nitric acid (HNO₃) is another powerful leachant for tin, given its strong oxidising nature. It can rapidly convert metallic tin to tin oxide/hydroxide. But as noted, this often yields a solid residue of SnO₂·H₂O instead of soluble tin.
A mixed acid approach, such as aqua regia or HCl–HNO₃ combinations, proves more effective. The combination leverages HNO₃ to oxidise tin and HCl to form soluble chloro-complexes. In the study by Castro et al., adding 1.0 N HNO₃ to 3.0 N HCl achieved ~98% tin extraction from ground PCB material3.
The challenge is avoiding interaction that causes reprecipitation – for example, residual nitric acid can oxidise Sn²⁺ to Sn⁴⁺ which, without sufficient chloride, may precipitate. Thus, careful washing or intermediate conditioning steps are usually employed.
Alkaline Leaching
Alkaline leaching offers a selective alternative for tin recovery. This process primarily uses strong bases, such as sodium hydroxide (NaOH) or potassium hydroxide (KOH), to dissolve tin in its +4 oxidation state (Sn⁴⁺), forming soluble stannate (Na₂[Sn(OH)₆] or K₂[Sn(OH)₆]).
The main advantage of alkaline leaching over acidic processes is its selective dissolution of tin and lead, while leaving behind valuable metals like copper and gold, which are less soluble in alkaline solutions.
Recent studies have focused on improving the efficiency of alkaline leaching by introducing various oxidants and modifying process parameters. For example, researchers have explored the use of peroxides (H₂O₂) or air oxidation to enhance the oxidation of tin to its +4 state. This combination of oxidants with alkaline solutions has shown promising results in improving the recovery rates of tin.
Hydrometallurgical techniques, however, present several challenges. First, the use of strongly acidic or alkaline reagents can cause significant corrosion to equipment, necessitating the use of more expensive corrosion-resistant materials. This not only increases operational costs but also introduces safety risks for operators.
Additionally, the high cost of reagents makes the process less economically viable. The low selectivity of acidic leaching complicates the isolation of individual metals, reducing efficiency.
Furthermore, hydrometallurgical leaching generates toxic off-gases, requiring costly scrubbing systems for proper treatment. The process also produces large volumes of hazardous waste, which can be difficult and expensive to dispose of safely.
Finally, maintaining precise pH control is crucial to prevent the unwanted precipitation of tin, which can be challenging to manage effectively.
Pyrometallurgical
Pyrometallurgical processing uses high temperatures to liberate and separate metals. In e-waste recycling, pyrometallurgy encompasses methods ranging from incineration (burning to remove organics) and smelting (melting with reductants/fluxes) to novel techniques like flash Joule heating.
Incineration & Pyrolysis
Incineration involves burning e-waste in the presence of oxygen, primarily to oxidise and remove organic substances (plastics, resins, etc.) and concentrate metals in the remaining ash.
Pyrolysis is a similar thermal treatment but carried out in an oxygen-deficient atmosphere, causing organic components to decompose into char, oil, and gas rather than fully combust.
Both incineration and pyrolysis serve as pre-treatment steps that reduce the volume of e-waste and produce a metal-rich residue, though by themselves they do not refine metals.
After incineration/pyrolysis, tin is left behind mostly as oxide or metal particles mixed with other ash constituents. Modern incinerators include off-gas filtration systems to capture any metal-bearing dust or vapor.
The end product of incineration is an inert slag/ash that contains the metals (tin, copper, precious metals, etc.) in oxidised form. This ash can then be sent to smelting or leaching processes for metal extraction.
Incineration thus helps by removing plastics and other combustibles that would otherwise complicate chemical leaching or mechanical separation. It also can generate energy: e-waste calorific value is high due to plastics, so incineration can be coupled with energy recovery (electricity or steam generation).
However, incineration must be carefully managed to avoid emission of toxic compounds – many electronics contain brominated flame retardants and chlorine from PVC, which can form dioxins and furans upon burning. High-temperature, oxygen-rich conditions and flue gas treatment are employed to minimize these emissions.
Smelting
 Smelting is the core pyrometallurgical method for metal recovery from e-waste and is already used at industrial scale to recover tin (among many other metals).
Smelting is the core pyrometallurgical method for metal recovery from e-waste and is already used at industrial scale to recover tin (among many other metals).
In smelting, e-waste (often after shredding and removal of some components) is melted in a furnace at high temperatures. The goal is to segregate metals into a molten metallic phase and a molten slag phase.
By adding appropriate fluxes and reductants, the smelting process is tuned so that valuable metals (copper, tin, precious metals, etc.) collect in the metal alloy (“black metal”) at the bottom of the furnace, while oxides of less valuable elements go into the slag.
Tin’s behaviour in smelting is somewhat complex: tin oxide (from solder) can be reduced to metallic tin by carbon and dissolve into the copper/lead bullion, but tin can also form oxide compounds that report to the slag or fume off if not sufficiently reduced.
Industrial smelters mitigate losses by operating under slightly reducing conditions and by capturing furnace off-gases.
In summary, smelting is a proven pyrometallurgical route for tin: it can efficiently extract tin into metal phases, but usually in combination with other metals.
The output requires further refining to isolate tin in marketable form, and any tin trapped in slags/dusts may need additional processing (or can be sold for recovery in other facilities).
Flash Joule Heating
Flash Joule heating is an emerging technique pioneered in recent years (notably by researchers at Rice University) for treating e-waste.
In this process, a high-voltage electric discharge through shredded e-waste powder raises its temperature by ~3,400 K within milliseconds. Essentially, the material is “flashed” into an extremely hot state momentarily.
This rapid joule heating drives off organic components and vaporises many metals, which then re-solidify as fine particles or condense on the reactor walls. The result is a metal-enriched residue that can be easily leached for metal recovery.
This method is being trialled commercially by an Australian company MTM Critical Metals. Importantly, flash Joule heating is far less energy-intensive than conventional furnace smelting. This makes it a promising cost-effective and eco-friendly pyrometallurgical approach.
While pyrometallurgical methods are effective for processing large volumes of e-waste, they require high energy input and often result in higher emissions. Additionally, further refining processes are typically necessary to extract pure tin.
Despite these challenges, pyrometallurgy offers a promising solution for the sustainable recovery of tin, especially as demand grows.
Next generation innovations
Deep eutectic solvents (DES)
 Deep eutectic solvents can be described as mixtures of solvents that form a eutectic mixture with a lower melting point than any of the individual solvents. These mixtures can be tuned by altering ratios and concentrations of solvents to alter its properties. DES’s are more environmentally friendly as the use less toxic and corrosive acidic/alkalis.
Deep eutectic solvents can be described as mixtures of solvents that form a eutectic mixture with a lower melting point than any of the individual solvents. These mixtures can be tuned by altering ratios and concentrations of solvents to alter its properties. DES’s are more environmentally friendly as the use less toxic and corrosive acidic/alkalis.
This methodology has been trialled in academic literature. A collaboration between the Indian Institute of Technology Delhi and Leeds University have explored using green choline chloride-based eutectic solvents to extract metals from thermally treated mobile phone PCBs. Oxalic acid, found in spinach, is able to selectively leach tin from the PCB within the eutectic mixture.
Formic acid-choline chloride selectively leached copper, iron and nickel, whilst urea-choline chloride successfully leached zinc. 92% of the tin concentrated on the PCB was successfully leached in just 1 hour at 80°C4.
This highlights a green, low toxicity, low energy method to recover tin from e-waste. This method is not only being used academically, but also commercially. UK based DEScycle is using deep eutectic solvents to recover metals including tin from e-waste. In fact, they raised over £10 million in Series A funding to commercialise the process.
This highlights that DES’s are ideal for a low environmental impact recycling process, and that the scalability of the process is being explored.
Organic Acid Leaching
Leaching using more environmentally friendly agents, such as organic acids, can yield similar tin recovery rates without the toxic emissions, waste and costs that come with other leaching agents.
Once study from researchers in China used citric acid, commonly used as a flavouring additive, as a leaching agent, and ascorbic acid as a reducing agent, to effectively leach tin from e-waste. The organic acid system combined with ultrasonic waves enabled a high tin recovery rate of 89%5.
Bioleaching
 Bioleaching uses living organisms to extract metals from circuit boards without the need for toxic chemicals. It also reduces the production of hazardous waste that can be difficult to dispose of safely. Usually pre-processing of e-waste is required such as shredding or crushing.
Bioleaching uses living organisms to extract metals from circuit boards without the need for toxic chemicals. It also reduces the production of hazardous waste that can be difficult to dispose of safely. Usually pre-processing of e-waste is required such as shredding or crushing.
A collaboration between Indian and Ethiopian universities has investigated using Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans to leach metals from powdered PCBs. After 28 days at 31°C over 96% of Sn2+ was recovered successfully6. The challenges addressed from this academic paper identified scalability as a potential drawback for this process, as well as leaching time.
However, bioleaching is being trialled commercially by a UK-based company Bioscope. This identifies that bioleaching processes are scalable and identifies a key opportunity for the green recovery of metals from e-waste.
Innovative PCB designs
Another way to combat the issues with recycling e-waste is adapting the design of PCBs themselves. This is being explored by Jiva Materials.
Jiva Materials is a UK based company that specialises in their soluboard design, which is a PCB made of materials that are dissolvable in hot water. Their soluboard has a 67% lower carbon footprint compared to glass-fibre and epoxy technologies.
Additionally, Swiss based company HyPElignum are exploring the creation of wooden PCBs. This is a carbon neutral process and enables higher recyclability of PCBs.
However, the cost to manufacture more sustainable PCBs can sometimes make them less competitive to existing designs. Also, biodegradable circuit boards need to be designed to endure heat and moisture effectively. Therefore, further design optimisation is needed to ensure that biodegradable circuit boards are suitable and competitive to existing designs.
Key players in e-waste recycling
The e-waste recycling market is being populated worldwide. Companies are adopting a variety of methods to selectively recover metals from e-waste.
For example, companies such as Bioscope in the UK are using bioleaching technologies. MTM Critical Metals in Australia is using flash joule heating technologies, and the SCARCE alliance between the CEA in France and Nanyang Technological University in Singapore are using SMART sorting techniques to successfully recover metals from e-waste.
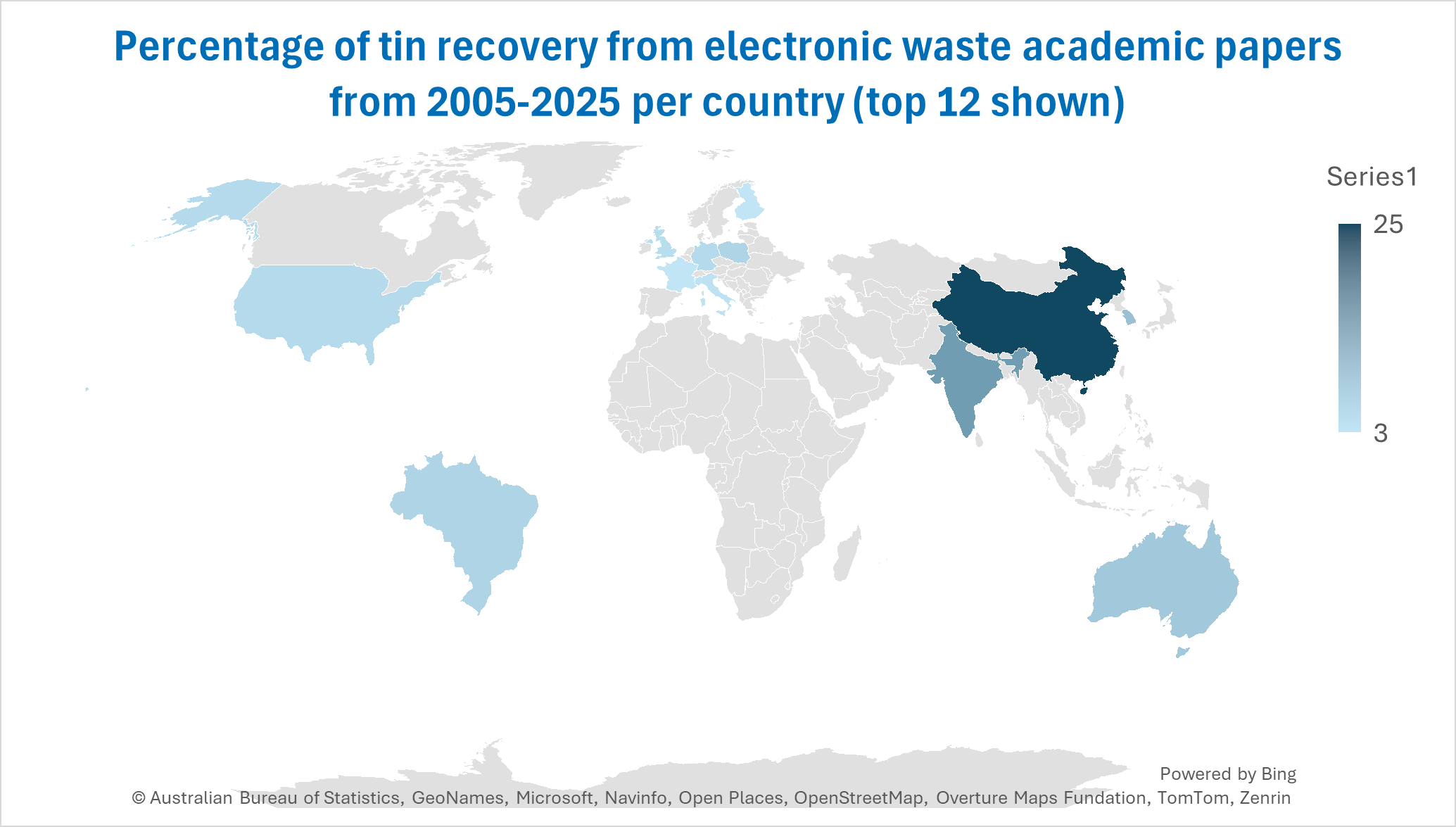
Universities worldwide are increasingly focusing on this topic. As shown in the figure, China leads the way, accounting for 25% of academic papers on tin recovery from electronic waste. India follows with 13%, while South Korea closely trails at 8%. This underscores the significant concentration of e-waste recycling research in Asia, the region with the highest levels of e-waste production.
In contrast, research on e-waste recycling in Africa is limited, largely due to challenges such as insufficient funding and infrastructure for recycling within the region. This presents a clear opportunity for the establishment of an African e-waste recycling facility, which could help transition from current crude recycling methods that have severe negative impacts on both health and the environment.
E-waste recycling regulations
In the EU a regulation related to the destruction of persistent organic pollutants (POPs) has been adopted. POPs are chemical substances that do not break down easily and thus can accumulate and risk being harmful to both human health and the environment. POPs are often found in e-waste.
POPs need to be destroyed thermally according to EU regulations during e-waste recycling to minimise their risk to human health and the environment.
Future outlook
The future of tin recovery from e-waste is poised for significant advancement driven by technological innovation, evolving regulations, and a growing global need for sustainable materials. As the e-waste stream continues to rise alongside the increasing demand for electronics, particularly in sectors like renewable energy and electric vehicles, effective recycling methods will become even more critical. The following trends and developments are expected to shape the landscape of tin recovery from e-waste:
Technological advancements
Traditional methods such as hydrometallurgy and pyrometallurgy will likely remain integral to tin recovery, but they will increasingly be complemented by more sustainable and efficient processes. Green methods, including deep eutectic solvents (DES) and bioleaching, are gaining traction due to their lower toxicity and environmental impact.
The adoption of cutting-edge sorting technologies will be crucial to improving the efficiency and effectiveness of e-waste recycling. Techniques such as X-ray fluorescence (XRF) and convolutional neural networks (CNNs) for material identification are enhancing the precision of sorting, enabling the recovery of valuable metals like tin at much higher rates. These advancements will likely become more widespread as automation in e-waste processing plants continues to evolve, leading to more streamlined operations with fewer environmental hazards.
Innovations in PCB design, such as the development of dissolvable and biodegradable circuit boards, represent a pivotal shift toward sustainability in electronics manufacturing. These advances in PCB design, while still in early stages, suggest that future electronics could be far easier to recycle, potentially reducing the need for complex extraction processes.
Regulatory, social, environmental and economic pressures
As e-waste volumes continue to increase, the need for robust recycling infrastructure and stricter regulations will become more pronounced. Regions like Europe and the Americas have already implemented Extended Producer Responsibility (EPR) policies, but such regulations will likely spread globally, especially in high e-waste-producing regions. The increased push for eco-design principles, coupled with national and international legislative support, will accelerate the adoption of responsible e-waste recycling practices. This will not only drive innovation in recovery technologies but also create a level playing field for e-waste management across different regions.
As e-waste recycling efforts in developing regions like Africa and Southeast Asia grow, significant opportunities for infrastructure development and research will emerge. The current lack of formal e-waste recycling infrastructure in these regions presents both a challenge and a significant opportunity. Investment in advanced recycling technologies and facilities could enable these regions to eliminate outdated, harmful practices, directly addressing health and environmental concerns. Furthermore, the rising awareness of sustainable practices and the potential economic benefits of recycling could lead to a shift in regional policies, opening doors for global collaborations in e-waste management.
The increasing reliance on tin in emerging technologies such as sodium-ion batteries, solar panels, and CO2 capture underscores the importance of a reliable, sustainable source of this critical material. The recovery of tin from e-waste presents a unique opportunity to supplement traditional mining, potentially easing the pressure on global tin supply chains. As demand for tin in high-tech applications grows, the economic viability of tin recovery from e-waste will become increasingly attractive, driving further investment in recycling technologies and infrastructure.
Conclusions
The future of tin recovery from e-waste looks promising, with significant advancements expected across technological, regulatory, and economic spheres. The integration of sustainable methods, coupled with the scaling of advanced recycling technologies, will drive more efficient recovery processes while minimising the environmental impact of e-waste. As the global demand for tin rises, especially in high-tech applications, e-waste recycling will become a critical part of the supply chain, creating a more sustainable and circular economy.