Technical Summary
Introduction
 The growing demand for sustainable energy solutions has driven the development of various energy storage technologies. Since their introduction in 1991, lithium-ion batteries (LIBs) have become a widely used energy storage option due to their high energy density. However, lithium is a relatively rare metal, making up less than 0.01% of the Earth’s crust. This scarcity has led to increasing prices, raising the cost of LIBs. Additionally, LIBs are associated with safety risks, such as thermal runaway, which can result in dangerous fires.
The growing demand for sustainable energy solutions has driven the development of various energy storage technologies. Since their introduction in 1991, lithium-ion batteries (LIBs) have become a widely used energy storage option due to their high energy density. However, lithium is a relatively rare metal, making up less than 0.01% of the Earth’s crust. This scarcity has led to increasing prices, raising the cost of LIBs. Additionally, LIBs are associated with safety risks, such as thermal runaway, which can result in dangerous fires.
These challenges have fueled the search for new, safer, and more affordable alternatives to LIBs. Sodium-ion batteries (SIBs) have emerged as a promising alternative. Sodium, the sixth most abundant element in the Earth’s crust, is cheaper and more widely available than lithium, making it an attractive option for large-scale energy storage. Furthermore, SIBs do not face the same thermal runaway risks as LIBs, making them safer.
However, SIBs still require further development to match LIBs in energy density. Key areas of focus include optimizing the materials for the anode, cathode, and electrolyte and improving overall battery design.
One active research area is using tin as an anode material in SIBs. In its pure form and combined with other elements, tin has gained attention due to its significantly higher theoretical capacity (~847 mAh g-1) than the commonly used hard carbon anode (~300-400 mAh g-1). This increased capacity is attributed to tin’s ability to form alloys with sodium, which could potentially enhance the energy density of SIBs. While most research has concentrated on tin’s role in the anode, there is also exploration into its potential in the cathode and electrolyte components. The main focus, however, remains on improving tin-based anodes to enhance cycling stability and energy efficiency as long-term performance challenges persist.
Operating principles
Sodium-ion batteries (SIBs) function similarly to lithium-ion batteries (LIBs), but with sodium ions instead of lithium ions. The key components of SIBs include the anode, cathode, electrolyte, separator, current collectors, and interfaces such as the solid electrolyte interface (SEI) and cathode electrolyte interface (CEI).
A comprehensive understanding of how each component works and behaves is essential for optimizing performance. The anode (negative electrode) in SIBs comprises materials capable of reversibly storing sodium ions.
Common anode materials include hard carbon, tin, and sodium. The cathode (positive electrode) is typically composed of sodium-containing compounds such as sodium cobalt oxide (NaCoO2), sodium iron phosphate (NaFePO4), or sodium manganese oxide (NaMnO2), and acts as the oxidising electrode during discharge phase.
During charging, sodium ions are released from the cathode and inserted into the anode (sodiation). In contrast, the ions move from the anode back to the cathode (desodiation) during discharge, generating an electric current that powers devices.
The electrolyte in SIBs usually consists of a sodium salt dissolved in an organic solvent. This allows sodium ions to move between the electrodes during charge and discharge cycles.
A separator, a permeable membrane between the anode and cathode, prevents direct contact between the electrodes while enabling ion flow. This separation is critical for avoiding internal short circuits and thermal runaway, thus enhancing the safety and stability of the battery.
The SEI is a thin passivating layer on the anode’s surface that forms during the first few charge-discharge cycles. It results from reactions between the electrolyte and the anode material and is crucial for protecting the anode from further degradation. The SEI permits sodium ions to pass through but prevents further electrolyte decomposition. However, due to sodium’s larger ionic radius compared to lithium, the SEI in SIBs is more prone to instability, which affects cycling performance, lifespan, and efficiency.
The CEI, which forms at the interface between the cathode material and the electrolyte, also plays an important role in the electrochemical stability of the cathode and impacts battery performance. During charge-discharge cycles, the CEI undergoes dynamic changes, and its stability is key to reducing capacity fading and improving cycle life. While the SEI has been studied extensively, the stability of the CEI is equally important, especially as research increasingly focuses on enhancing the cycling stability of the cathode.
Current collectors, made from conductive and non-reactive materials, connect the electrodes to the external circuit. In LIBs, copper-based current collectors are typically used for the cathode, while aluminium is commonly used for the anode. In SIBs, aluminium current collectors are often used at the anode, offering a more cost-effective solution and making SIBs more suitable for large-scale applications.
Benefits of tin use
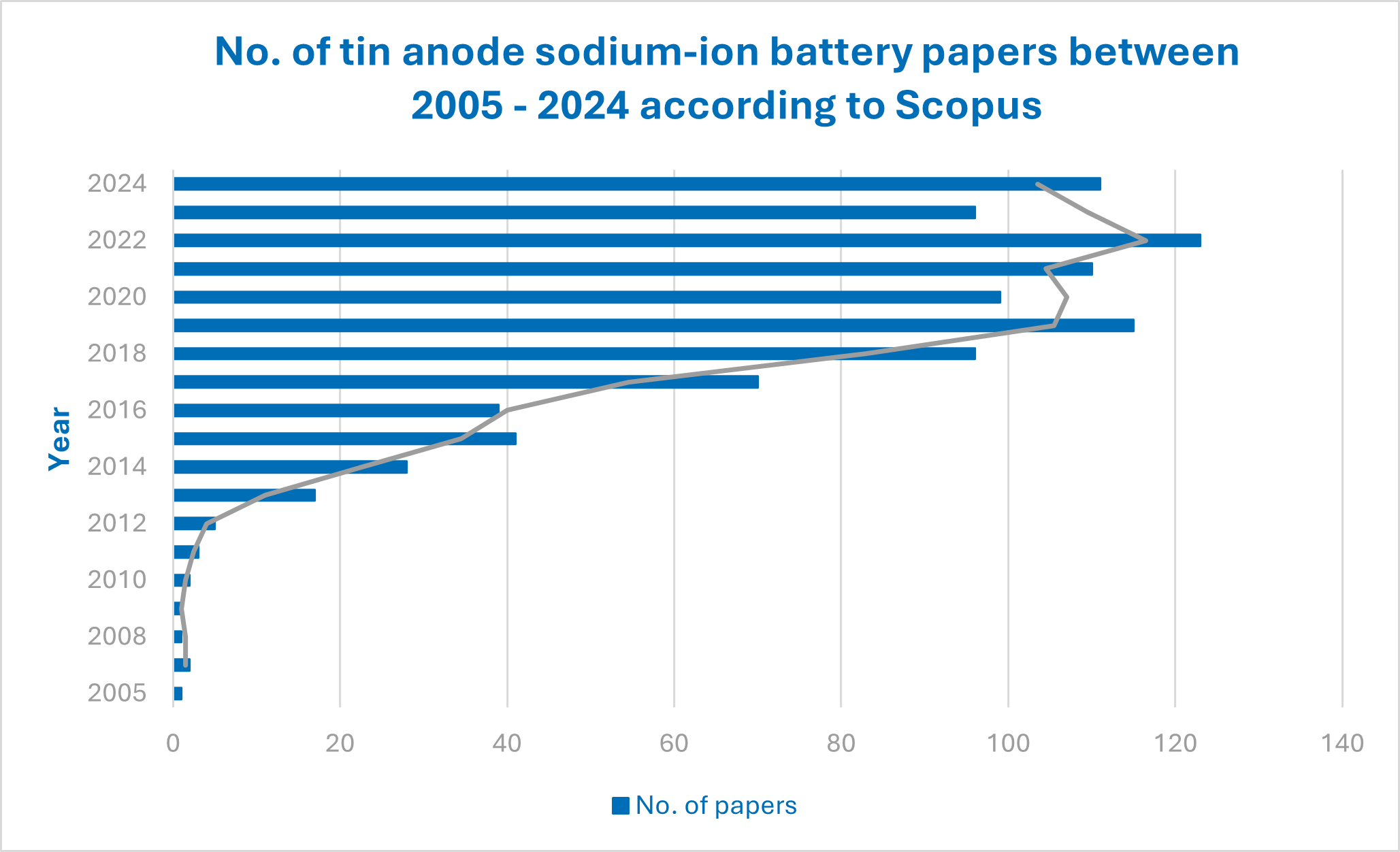
Tin has multiple applications inside of an SIB including as a potential cathode and electrolyte material. Its main application, however, is within the anode material. Academic papers on this topic have been growing, particularly over the last decade.
Start-ups such as Nanode and Unigrid are attracting funding, and existing SIB battery companies are also evaluating tin-based anodes for improved battery performance.
Tin has already demonstrated potential as an anode material due to the ability of its metallic form to alloy with sodium forming Na15Sn4, resulting in a high theoretical capacity of 847 mAh g-1. Compared, most hard carbon-based anodes show a theoretical capacity of around 300 mAh g-1.
Tin’s high volumetric density enables smaller, lighter batteries with the same amount of power, ideal for applications such as EVs. Tin is an abundant element, easy to handle and compatible with low technology production routes.
Technical issues to solve
Although tin has many advantages as an anode material, areas of improvement are needed to enable long-lasting, powerful batteries.
Tin undergoes severe volume expansion (up to 400%) during the alloying process with sodium ions. This is caused by sodium ions being much more significant in atomic volume than tin, so the structure is forced to expand when the sodium ions intercalate into the tin lattice. This is a non-reversible process and can lead to short battery life.
Additionally, the SEI layer inside batteries is a crucial protective layer preventing tin-electrolyte reactions that cause capacity loss. However, the SEI layer can be cracked during volume expansion, allowing unwanted side reactions. This increasing capacity loss can affect the overall performance and lifetime of the battery.
Furthermore, the electrolyte material may need to be carefully selected when using tin-based anodes. Some forms of tin are reactive and, if not compatible, can form a thick, non-conductive and unstable SEI layer. This can be true with conventional carbonate-based electrolytes. Tin’s speciation and migration profile may need to be better understood, or alternative electrolyte materials may need to be used.
Technical solutions
Volume expansion can be resolved in different ways using well-known approaches from studies of tin and silicon used in lithium-ion battery research.
Due to its increased surface area and more flexible structure, nanostructured tin can help accommodate the expansion.
Combining tin with other materials, such as hard carbon, can help buffer the volume changes by supporting the tin particles on or within a stable matrix. Universities researching tin-carbon composite anode materials include the University of St Andrews, Imperial College London, National University of Sciences and Technology Pakistan, and Northumbria University.
Alloying tin with other elements such as antimony, phosphorus, or sulfur can also help buffer volume expansion. Researchers at National University of Sciences and Technology Pakistan are studying cobalt and sulfur integration with tin and Macau University of Science and Technology are also looking at cobalt-tin anodes.
Glyme-based electrolytes may be more compatible with tin-based anodes. Glyme electrolytes tend to form thinner, more stable SEI layers and react less aggressively with tin.
Types of tin anodes
Several tin-based anode materials may be used in sodium-ion batteries (SIBs), each with unique advantages and disadvantages.
Metallic Tin anodes have the advantages of high theoretical capacity, easy sourcing, and inexpensive production compared to other alternatives. However, volume expansion and cycle life require specialised solutions.
Tin Alloys as anode materials may have additional advantages such as reduced volume expansion, cycling stability, and electrochemical performance due to better ionic diffusion pathways. However, they can have lower theoretical capacities than metallic tin, increasingly complex synthesis methodologies, and with some alloys poorer conductivity.
Tin Carbon composite anode materials have enhanced conductivity due to the conductive carbon network, reduced volume expansion, and improved cycle life. However, the overall battery capacity can be reduced, and synthesis routes can also be more complex.
Electroplated Tin on Copper has the advantages of improved conductivity, better adhesion, and structural integrity. It reduces the risk of delamination, although volume expansion still needs control. Since only a thin layer of tin is usually used, capacity may be reduced.
Tin Compounds, often offer benefits such as improved cycling stability and reduced volume expansion due to stabilisation from other elements such as sulfur. However, they typically have lower theoretical capacities than metallic tin, and their synthesis can be more complex. Additionally, their lower conductivity may limit performance, requiring further optimization.
Solid state batteries
Solid-state sodium-ion batteries (SSBs) use solid electrolytes instead of liquid ones, offering enhanced safety, thermal stability, and potential for higher energy densities. The solid electrolytes, typically inorganic ceramics or sulfides, have lower ionic conductivity than liquid electrolytes, requiring careful design to ensure good power output and cycle life.
While solid-state systems can suppress dendrite growth and improve safety by preventing electrolyte leakage, the main challenge is achieving stable ionic contact and maintaining high efficiency over many cycles. Recent research has made progress by using composite anodes and protective layers to address these issues, showing promise for both grid storage and electric vehicle applications.
Performance metrics
The performance of tin anodes can be measured in multiple ways. For example, the specific capacity refers to the amount of charge that a material can store per unit of mass (mAh g-1), and it is measured at various current densities, which is the current flowing through a material relative to its mass (A g-1). As current density increases, a typical trend is the observed decrease in specific capacity. This occurs because higher current densities reduce the time available for ions to intercalate into the anode material, reducing the amount of charge stored per unit mass.
The published literature observes a general cluster of data points between 250-400 mAh g⁻¹ at lower current densities. This is consistent with the fact that much of the academic testing for these materials is likely conducted at lower current densities, where the performance of materials is typically higher due to more time for ion insertion and extraction.
High specific capacity at low current densities indicates that the material can store a large amount of energy in relation to its mass. This means the material has a high energy density, which is critical for applications requiring long run times or extended discharge periods.
Specific capacity at high current densities is vital for rate capability, which is its ability to charge and discharge quickly without significant loss of capacity. This is essential for high-power applications like EVs, power tools etc.
Tin and tin alloys
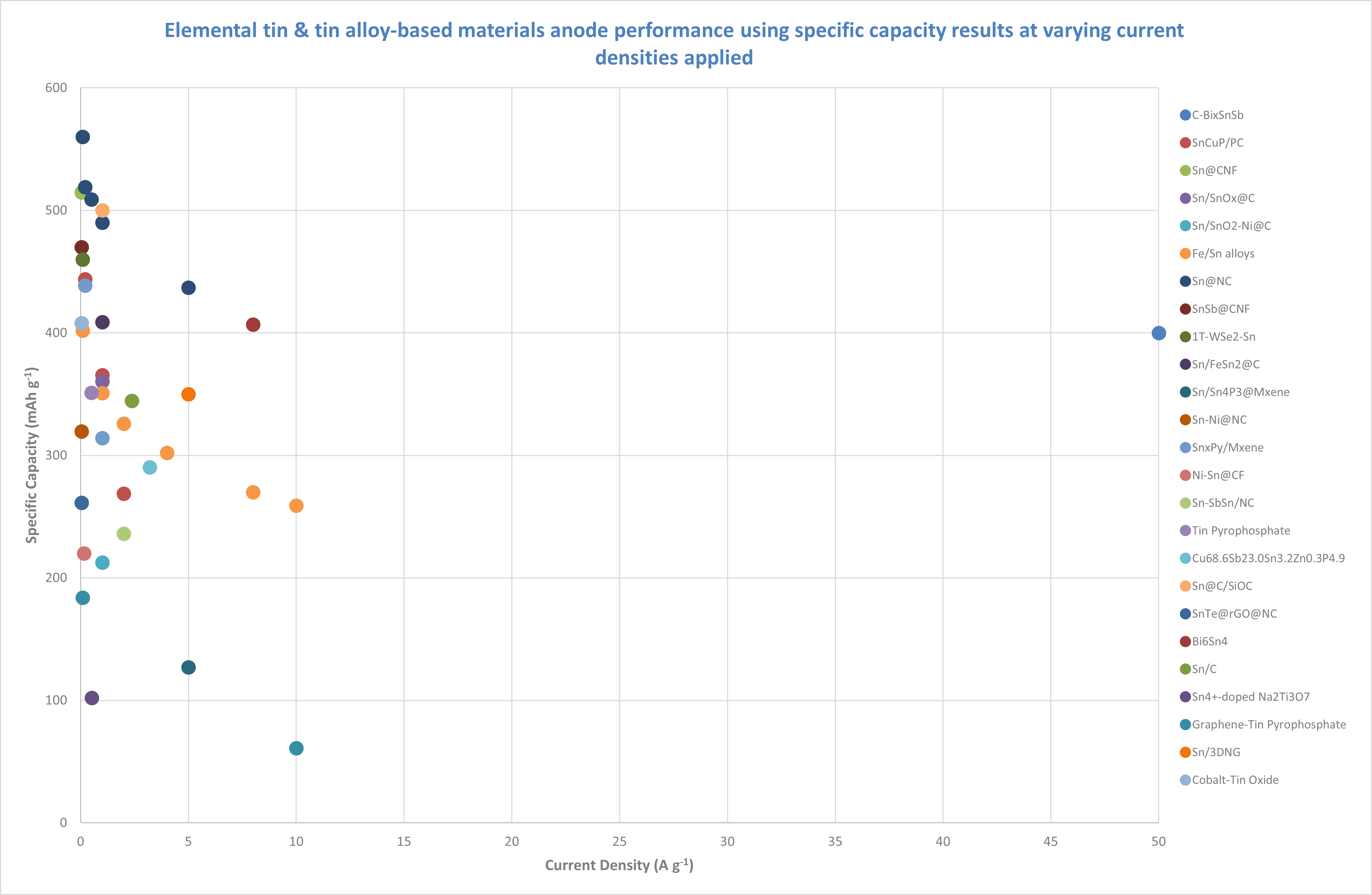
Most elemental tin and tin-alloy based anode materials have been tested at lower current densities, showing a wide range of specific capacities, from less than 100 mAh g⁻¹ to over 550 mAh g⁻¹. This diversity in results reflects the ongoing optimization efforts for these materials.
Sn@NC demonstrates the highest specific capacity at 0.1 A g⁻¹, with a 560 mAh g⁻¹ capacity. This material shows good stability across a range of current densities, with a 437 mAh g⁻¹ capacity at 5 A g⁻¹, signifying only a ~22% drop in capacity. The relatively small decline suggests that Sn@NC might be a stable material, with decent performance at higher currents, making it a strong candidate for applications requiring moderate power.
Fe/Sn alloy starts with a specific capacity of 402 mAh g⁻¹ at 0.1 A g⁻¹ and drops to 259 mAh g⁻¹ at 10 A g⁻¹, a ~36% drop in capacity. While this is a larger drop compared to Sn@NC, it’s important to note that the initial capacity is lower. Despite the significant drop, the Fe/Sn alloy still performs reasonably at higher current densities, especially considering the lower starting capacity.
Tin carbon
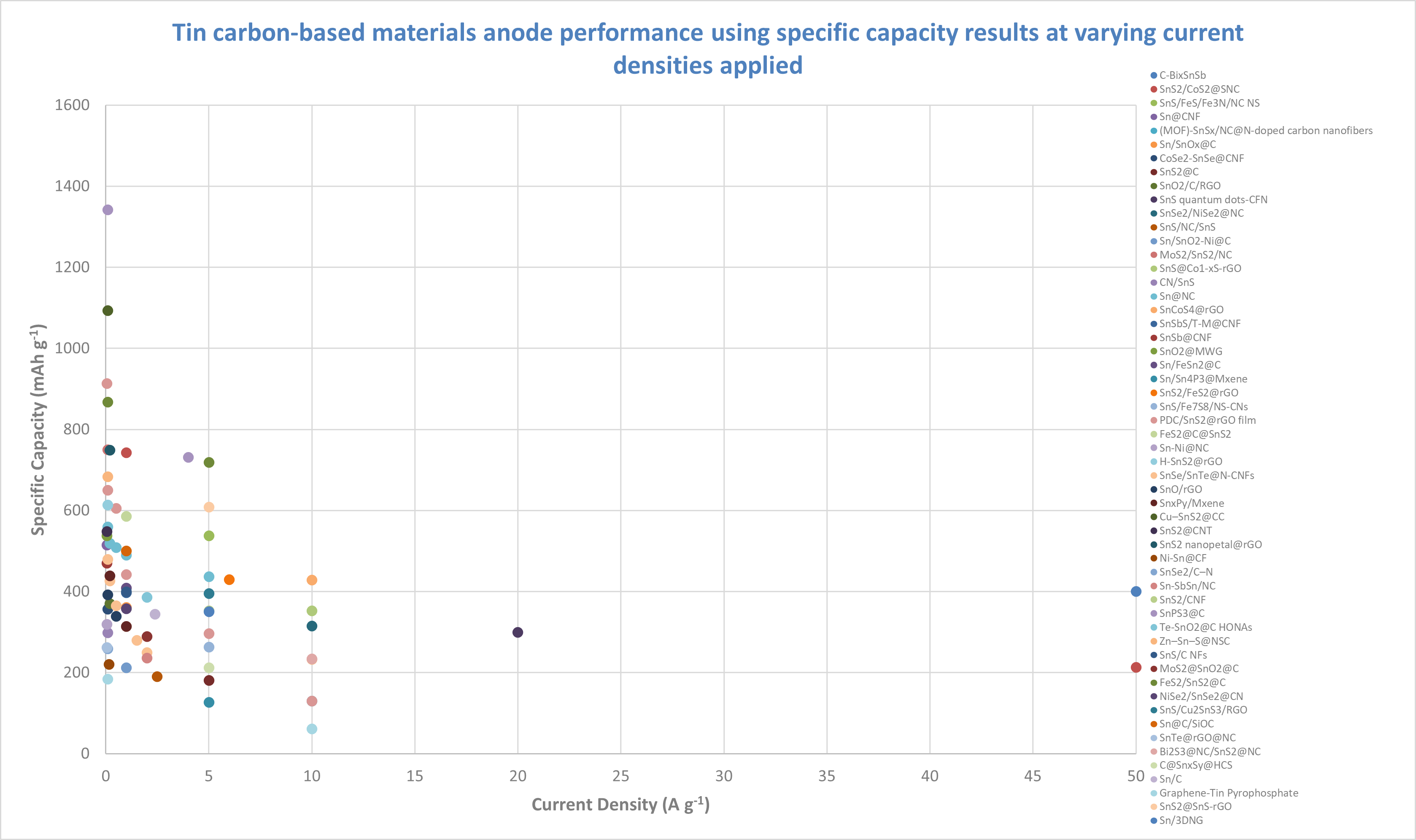
Many tin-based anode materials are being integrated with carbon, as seen in the graph’s variety of tin-carbon composite materials. This integration is likely beneficial for improving cycle life and enhancing stability. Carbon’s conductive properties and structural stability support tin during charge-discharge cycles, helping to mitigate issues like volume expansion and electrode degradation typically associated with tin anodes. C-BixSnSb shows the highest specific capacity (~400 mAh g⁻¹) at 50 A g⁻¹, indicating that it performs relatively well at high current densities. However, additional data points at lower current densities would provide a more complete picture of its potential at lower power demands.
SnS2/CoS2@SNC exhibits high specific capacity (~743 mAh g⁻¹) at 1 A g⁻¹ and performs reasonably well at higher current densities (~213 mAh g⁻¹ at 50 A g⁻¹), making it a potential candidate for applications requiring both energy and power density.
Tin oxides
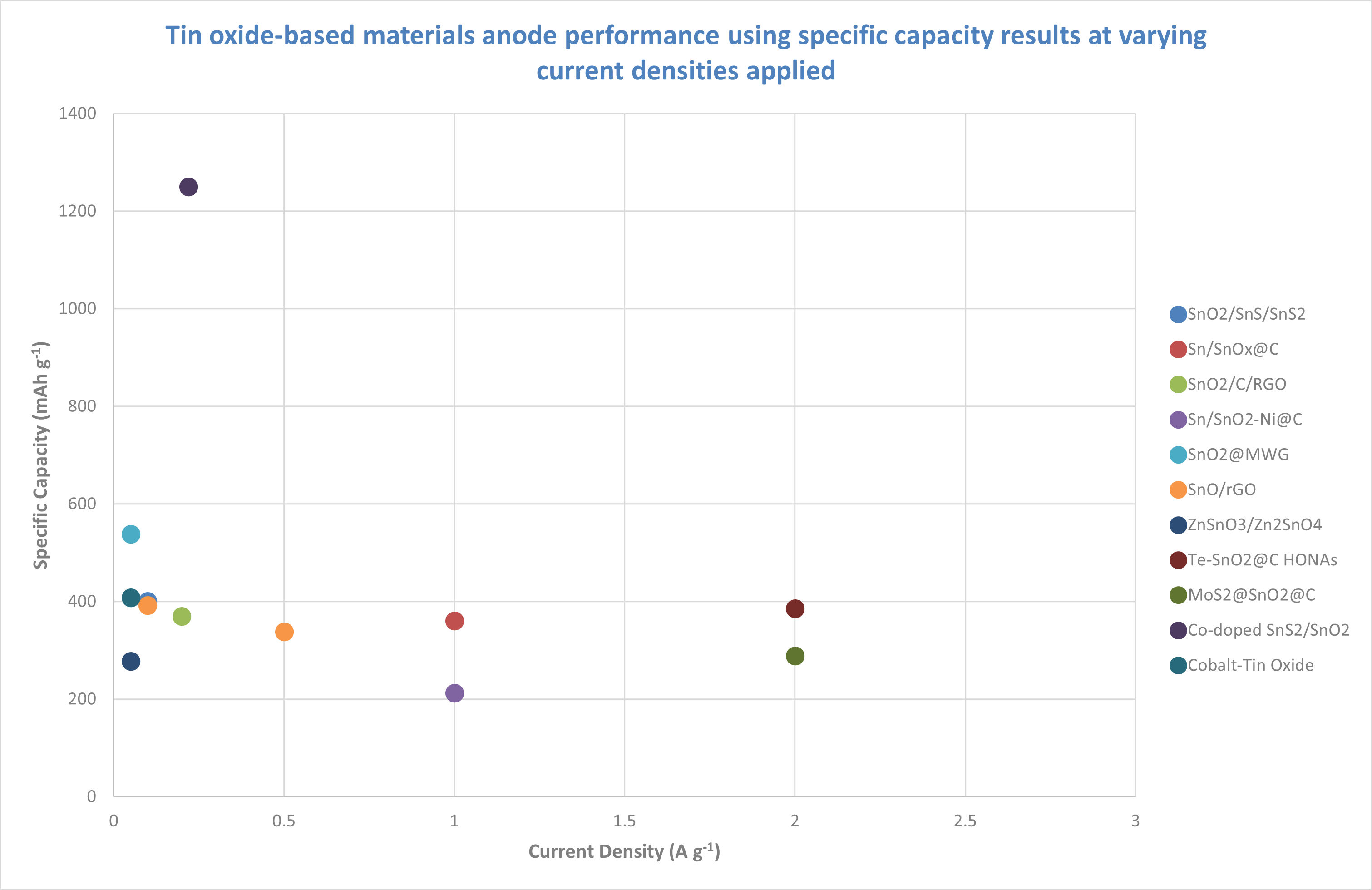
SnO₂/SnS/SnS₂ shows the highest capacity at low current densities, but more data is needed to evaluate its high-current performance.
Sn/SnO₂@C, SnO₂/C/rGO and similar materials show moderate capacity, which could be useful for moderate power applications.
Cobalt-Tin Oxide has the simplest formation but lowest capacity, making it preferable for lower power uses.
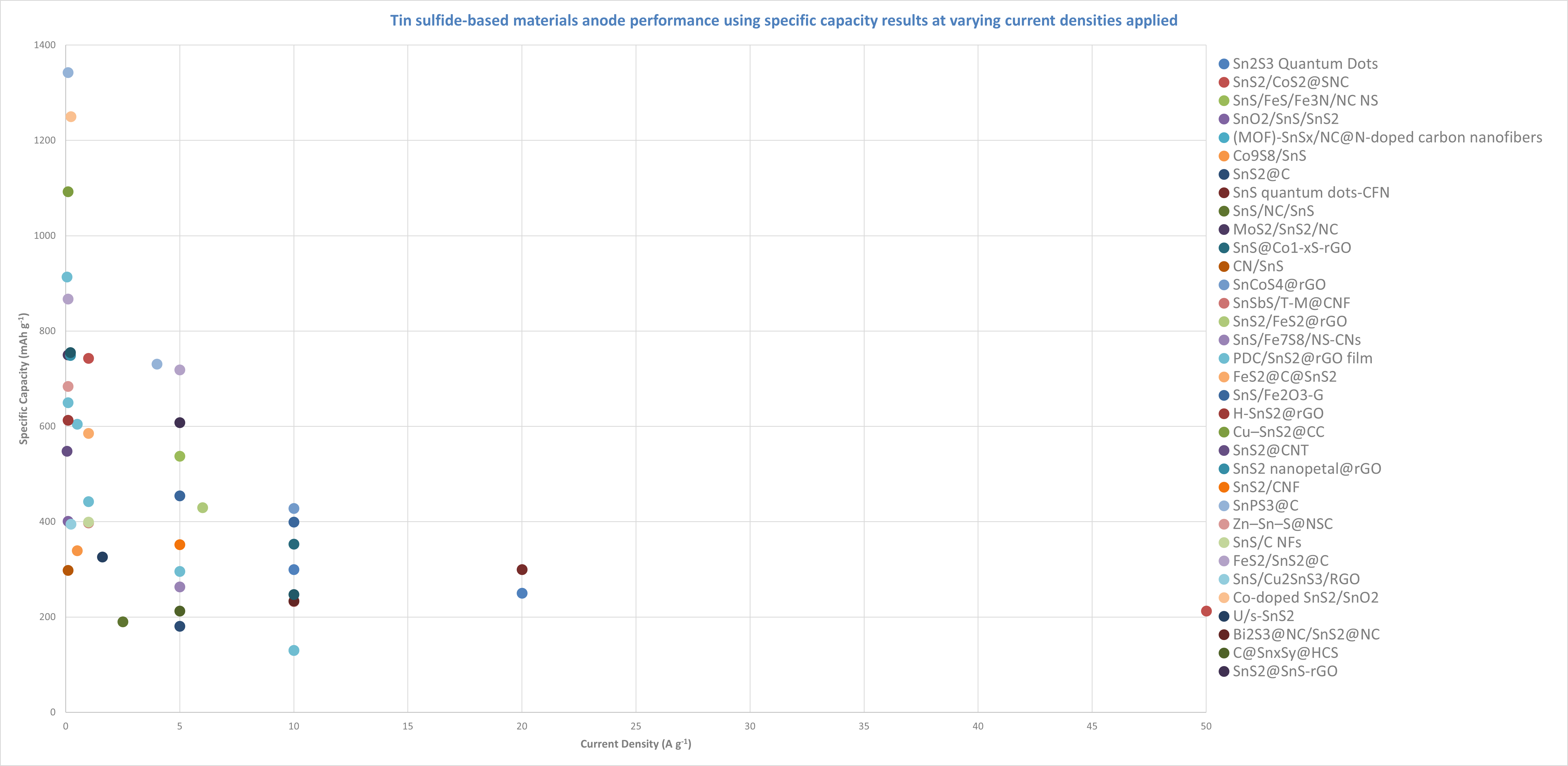
Tin phosphides and sulfides
Recent academic papers have explored a wide range of tin sulfide-based anode materials, indicating their growing importance and potential in energy storage applications. The variety of materials suggests that tin sulfides are considered promising candidates for enhancing battery performance, with ongoing research focused on optimising their performance.
SnPS3@C demonstrates the highest capacity in the references scoped (~1342 mAh g⁻¹) but at very low current density (0.1 A g⁻¹). However, as the current density increases to 4 A g⁻¹, its capacity drops to 731 mAh g⁻¹, representing a nearly 50% decrease in capacity. This significant drop is typical of many anode materials as current density increases, but SnPS3@C still maintains relatively high performance even at moderate current densities.
SnS2/CoS2@SNC is one of the few materials tested at very high current densities. At 1 A g⁻¹, it shows a capacity of 743 mAh g⁻¹. However, at 20 A g⁻¹, its capacity drops to 213 mAh g⁻¹, which represents a 70% decrease in capacity. Despite this dramatic reduction, the material’s performance at such a high current density is impressive and suggests good stability under high power conditions. However, it still experiences a notable decline in capacity.
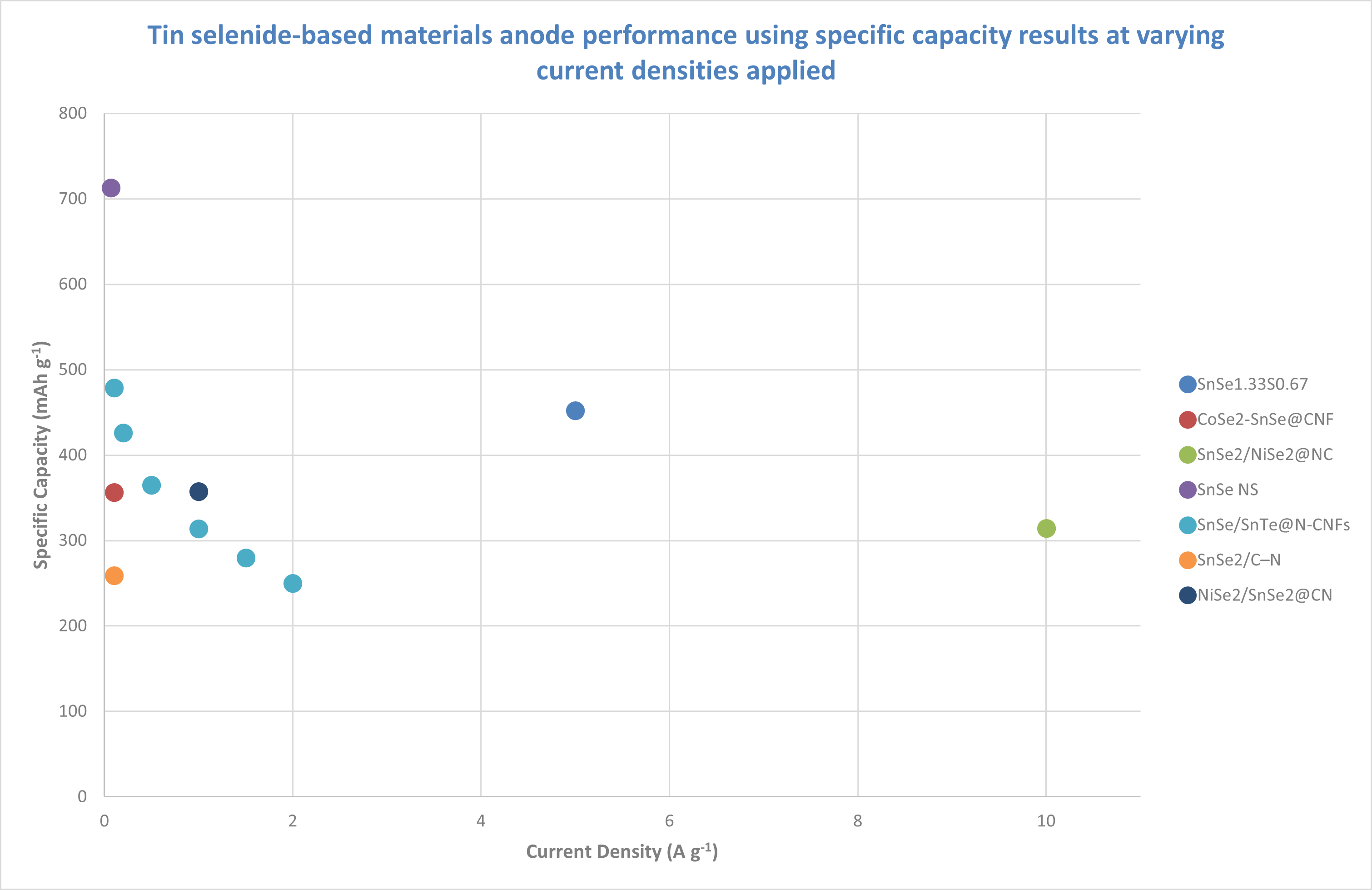
Tin selenide materials
This figure shows the performance of various tin selenide-based anode materials at varying current densities, as reported in recent academic studies.
Notably, the SnSe nanosheet array on carbon cloth (SnSe NS) exhibits the highest capacity at lower current densities, with values exceeding 700 mAh g⁻¹. This performance is significantly higher than that of most of the other materials observed in this study.
SnSe₂/NiSe₂@NC, despite high current densities (up to 10 A g⁻¹), maintains a specific capacity of around 300 mAh g⁻¹. This suggests that this material could exhibit improved stability and performance over multiple charge-discharge cycles. However, further tests, particularly regarding cycle life, would be required to confirm this behavior.
SnSe/SnTe@N-CNFs shows a noticeable drop in capacity with increasing current density, with data points mostly between 0.1 and 2 A g⁻¹. This rapid decline in capacity suggests that this material may not be suitable for high-power burst applications, where sustained performance at higher current densities is needed.
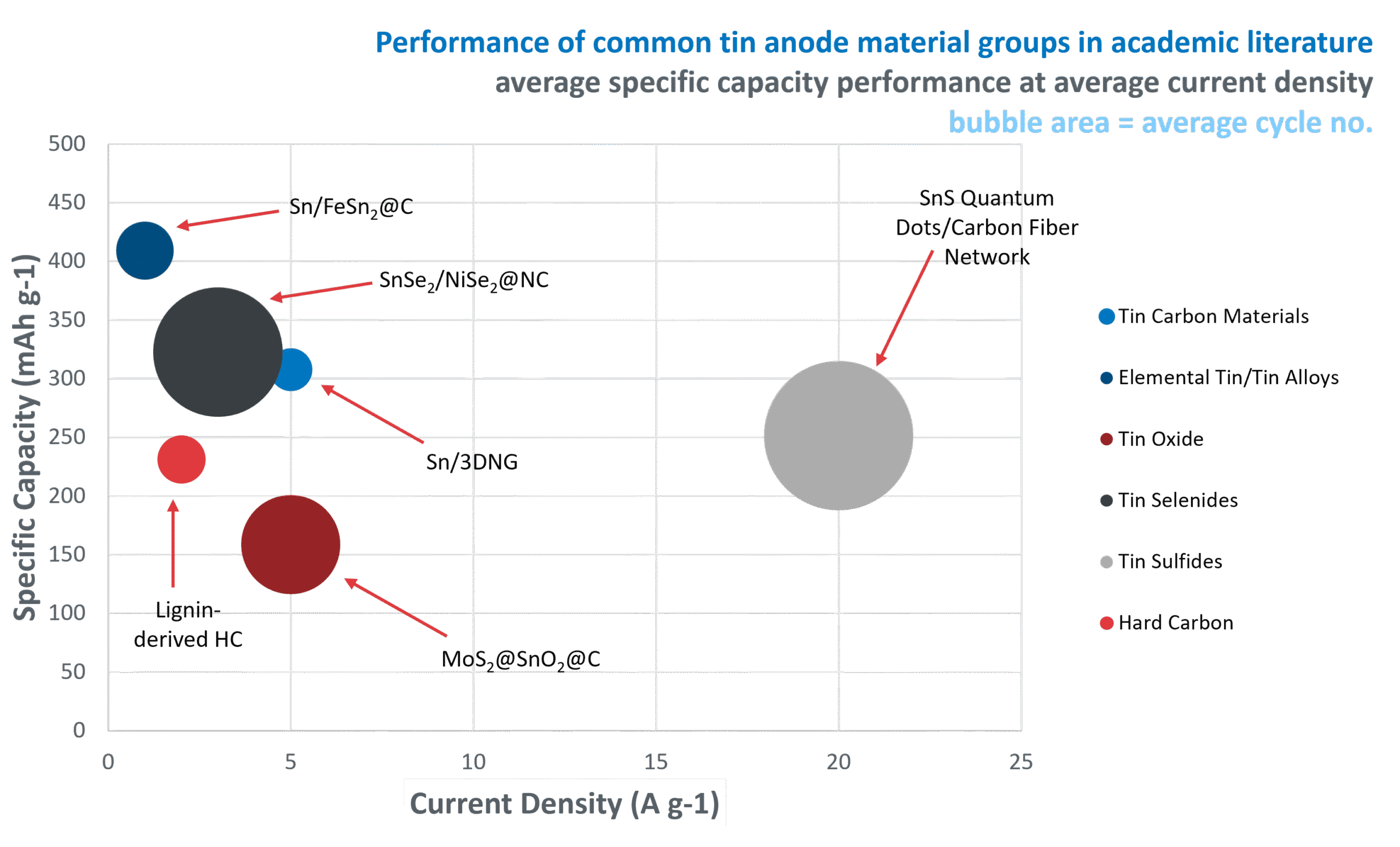
Cycle life
The figure highlights some of the most common tin research materials in the last year including tin selenides, tin sulfides, tin carbon materials, tin oxides and elemental tin/tin alloys. The graph highlights the relationship between specific capacity (mAh g⁻¹) and current density (A g⁻¹), with bubble size representing the number of tested cycles for each material in the literature. It is important to note that some materials testing at an academic level may be limited by time and equipment availability.
If we observe some of the results on the graph, tin sulfides appear to be a promising option. This specific example of tin sulfide quantum dots on a carbon fiber network has been tested at a high current density of 20 A g⁻¹ for 10,000 cycles whilst maintaining a specific capacity of over 250 mAh g⁻¹. Performing well at high current density means that the material is likely suitable for fast charging and discharging applications such as EVs. This is because at high current density, sodium ions have less time to diffuse across the electrolyte, but the material is still able to achieve a good specific capacity, meaning the anode material is suitable for fast ion transport. Additionally, given the material was tested for 10,000 cycles, it can withstand many charge/discharge cycles, identifying a long life span for the material, increasing the time length for battery replacements. It is also important to note that in recent academic literature, the most popular choice for tin anode materials has been tin sulfides.
Using the example materials chosen, we can also see that a form of carbon is being used in each anode material. Carbon can be integral to help stabilise tin anode materials, and buffer volume expansion during cycling.
On the graph we also have a hard carbon anode example. Its position on the graph highlights the opportunities for high capacity, longer lifetime, and faster charging capability when using tin-based anode materials.
Synthesis routes
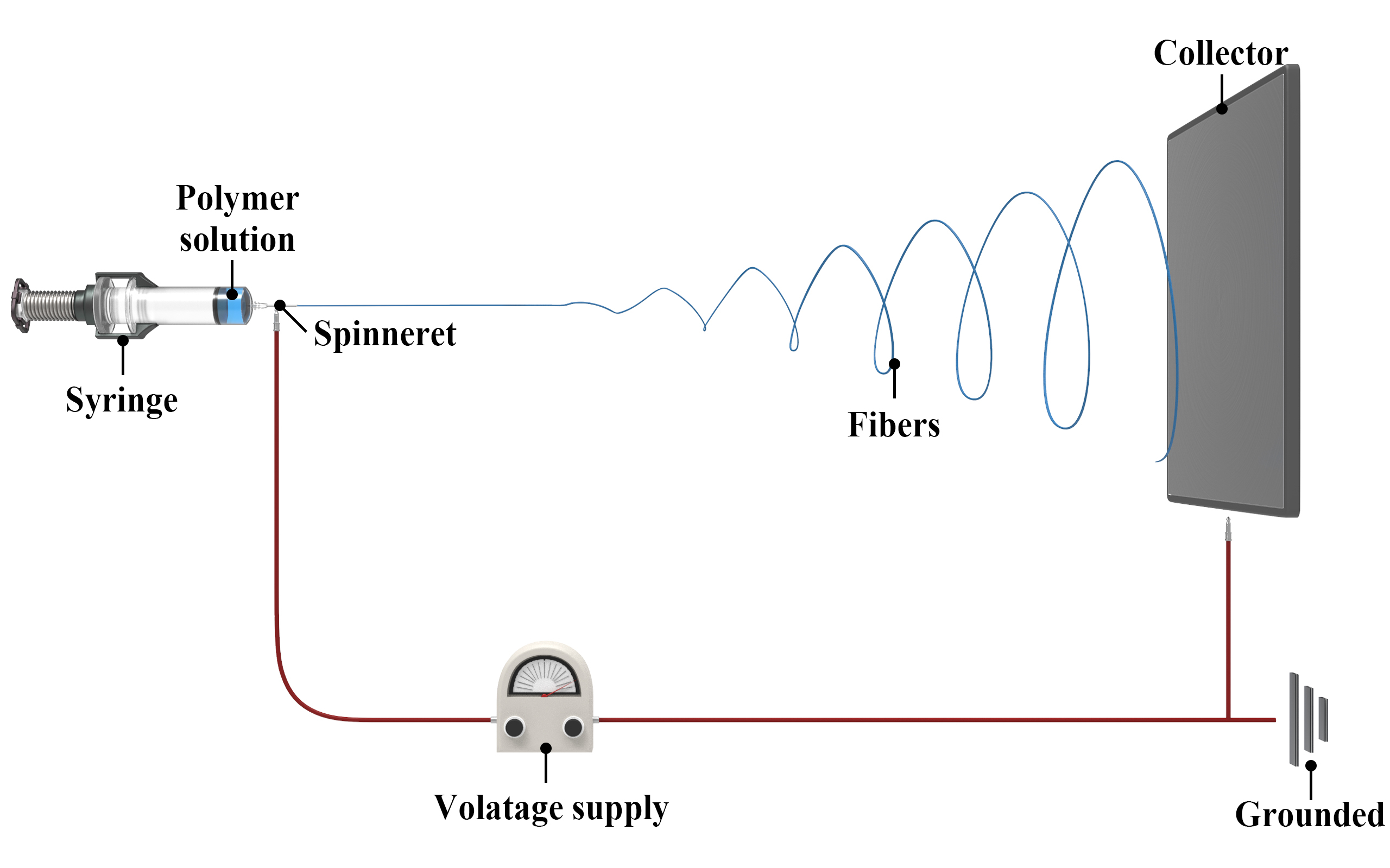 Electrospinning is a method that uses electrostatic forces to produce nanofibers with nanometre-scale diameters. A polymer solution, a tin precursor, and a solvent are combined, and the solution is loaded into a syringe with a metallic nozzle. A high voltage is applied, forming a Taylor cone, from which continuous nanofibers are drawn and collected on a grounded collector. The nanofibers can then be carbonised via heat treatment under an inert atmosphere, which removes organics and leaves behind tin embedded in carbon.
Electrospinning is a method that uses electrostatic forces to produce nanofibers with nanometre-scale diameters. A polymer solution, a tin precursor, and a solvent are combined, and the solution is loaded into a syringe with a metallic nozzle. A high voltage is applied, forming a Taylor cone, from which continuous nanofibers are drawn and collected on a grounded collector. The nanofibers can then be carbonised via heat treatment under an inert atmosphere, which removes organics and leaves behind tin embedded in carbon.
Solvothermal is a method that involves reactions in a closed system at moderate to high temperatures. For example, SnSe nanosheets on carbon cloth were created using a vacuum thermal evaporation method1. Electrochemical induction and an in-situ hydrothermal method have been combined to construct a multiphase structure of SnO2/SnS/SnS22. A one-step solvothermal method was used to prepare hierarchical flower SnS23.
Chemical vapour deposition (CVD) involves heating a chemical tin-based precursor to form a vapour, which then decomposes and reacts to form a thin film on a heated substrate. Physical vapour deposition (PVD), however, occurs under vacuum conditions where the tin source material is converted to vapour through physical means, which then condenses on the substrate surface to form a uniform thin film.
Wet chemical methods can be used for example to synthesise SnO nanoparticles involving oleylamine, tin chloride, and deionized water under an argon atmosphere, followed by heating, cooling, and washing steps4.
Ball milling is used to produce nanocomposites for example by Ren et al5. Additionally, ball milling was used to combine Fe and SnS2 mixed powders with graphite to fabricate FeS/SnS, FeSn2/FeS/SnS, and FeSn/FeSn2/FeS/SnS heterostructure composites anchored on exfoliated graphite6.
In-situ methods involve situ introduction of copper ions and a carbon conductive framework to form SnS nanocrystals embedded in a Cu2SnS3 lamellar structure heterojunction composite (SnS/Cu2SnS3/RGO) with graphene as the supporting material is proposed to achieve dual-driven sodium ion/electron migration during the continuous electrochemical process7.
Competitive anode materials
Hard carbon Hard carbon has the unique advantage of extremely low cost, and the opportunity to be derived from waste. It is also disordered in terms of structure, meaning it can accommodate sodium insertion much more effectively than other materials, avoiding volume expansion issues. Hard carbon can be combined with many other elements to enhance the anodes performance. It is also the most mature anode material for sodium-ion batteries, making it more advanced in terms of technology readiness level. However, hard carbon is limited by its lower reversible capacity of around 300 mAh g-1. Additionally, it has a low initial columbic efficiency (ICE) which means a lot of sodium is irreversibly lost in the first few cycles. It also has a significantly lower volumetric density than materials such as tin, meaning larger batteries are required which can be unfavorable for applications such as EV.
Bismuth Bismuth performs similarly to tin as an anode material. Bismuth is able to reduce volume expansion effects in comparison to tin (only ~250% expansion) which helps to improve battery cycle life. It is also a cheap material similarly to tin, as well as having a high volumetric density too. Bismuth is however limited by its much lower theoretical capacity of 385 mAh g-1.
Sodium Sodium has a very high theoretical capacity of 1166 mAh g-1. It is also a cheap resource, and one of the most abundant. It can even be harvested from seawater. However sodium anodes experience volume expansion (~200%) and dendrite formation where both largely decrease the cycle life of the battery. They are also typically not compatible with commonly used electrolyte materials.
Phosphorous Phosphorous has an incredibly high theoretical capacity of 2596 mAh g-1, which is significantly higher than previously discussed anode materials. It does however suffer from volume expansion issues (300%) and thus issues with battery cycle life are common. It also suffers from low electrical conductivity negatively affecting the rate capability of the battery. Additionally, whilst phosphorous is an abundant material, it is difficult and expensive to obtain pure high-quality black phosphorus used as an anode material.
Lead Lead has a reasonable theoretical capacity of 380 mAh g-1. Lead is also relatively abundant and low cost. Lead does however suffer from volume expansion and poor cycle life. It is also extremely dense making it less energy efficient per unit mass. Lead is a toxic metal with its environmental impact being a concern, making it a less attractive material option.
Transition metal phosphides (TMPs) TMPs typically have relative high theoretical capacities, for example nickel phosphide (NiP) has a theoretical capacity of 846 mAh g-1. This is thanks to their ability to undergo conversion and alloying reactions with sodium during charging and discharging. TMPs however do undergo volume expansion similarly to other anode materials. Additionally, phosphorous can be more expensive to obtain as previously discussed. TMPs can show loss in rate capability too due to slow diffusion rate of sodium ions through the TMP matrix, limiting performance.
Key players
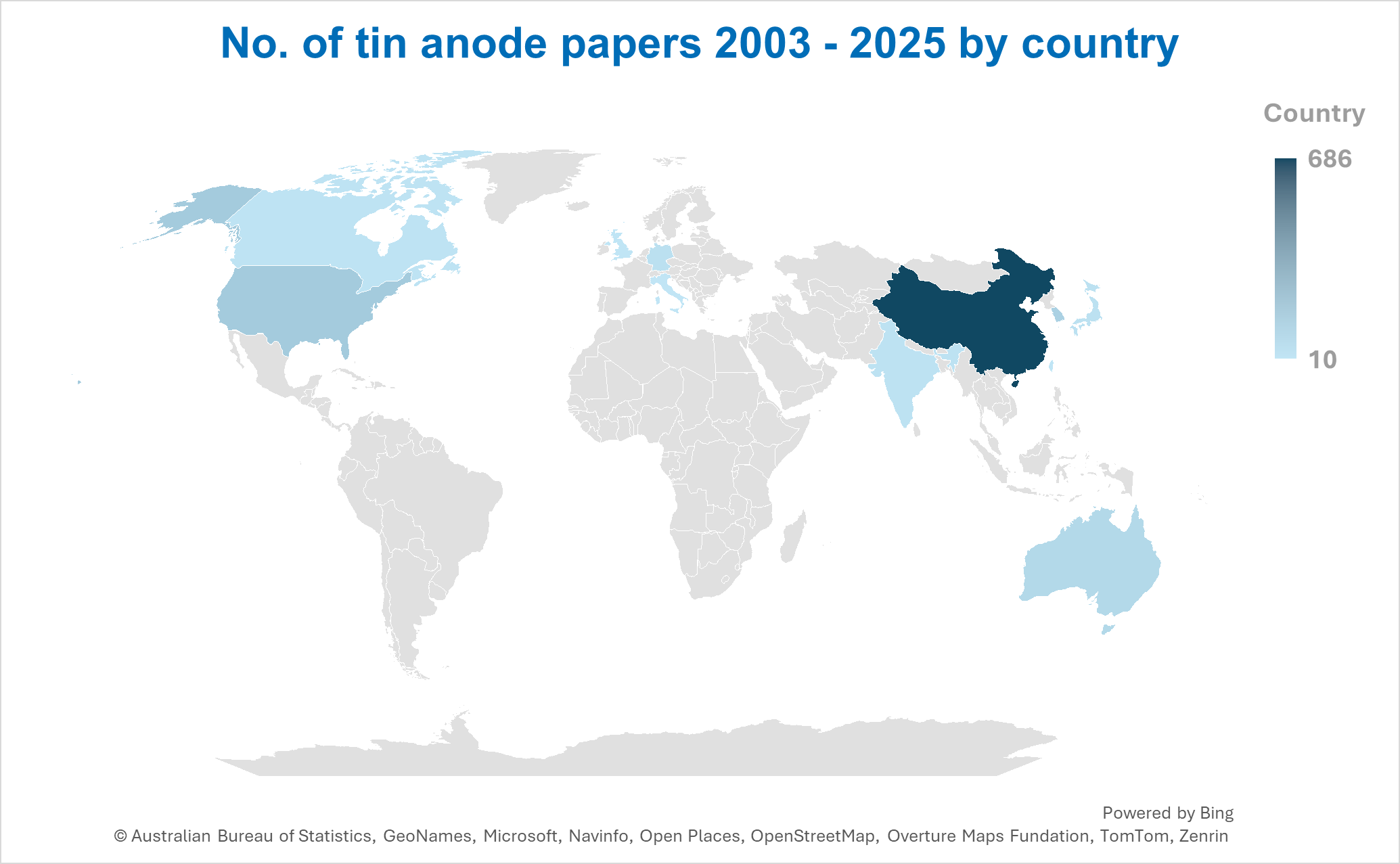
There are many academic institutions worldwide focusing on improving tin anode performance for sodium-ion batteries.
This figure breaks down the 987 tin anode papers for sodium-ion batteries created between 2003 to 2025 so far based on originating country.
The top 13 countries with the highest number of papers are represented.
China dominates representing almost 70% of total academic papers over the last two decades. This is followed by the United States with around 12%.
Several companies are making significant strides in advancing sodium-ion technology. U.S.-based Natron Energy is a notable player, having announced plans for a $1.4 billion investment in a manufacturing plant in North Carolina. This move will boost the company’s production capacity by 40 times, enabling it to produce 24 GW of storage annually at full capacity.
Similarly, China’s Contemporary Amperex Technology Co. Ltd. (CATL) is diversifying its product offerings, introducing its Freevoy battery, which combines sodium-ion and lithium-ion cells for extended-range hybrid vehicles. CATL’s commitment to enhancing sodium-ion technology is further exemplified by their second-generation sodium-ion battery, expected to launch in 2025, capable of operating at temperatures as low as -40°C.
The UK-based Faradion is another key player in the sodium-ion sector, recognized for its innovative technology and significant contributions to the market. In 2022, the company was acknowledged in the British Private Equity and Venture Capital Association’s Vision 2022 Initiative.
Meanwhile, HiNa Battery Technology, based in China, is utilizing sodium-ion technology in electric vehicles. The company’s partnership with JAC to power the Sehol E10X electric car with sodium-ion batteries marks a significant step for the industry, and one of the early sodium-ion battery powered vehicles.
Swedish company Altris AB is also making waves with its sodium-ion technology, which features a proprietary Prussian blue analogue cathode and hard carbon anode materials. Altris is collaborating with Clarios to bring its batteries to the market, demonstrating its commitment to advancing the field.
Established sodium-ion battery companies are looking to explore tin as an anode material to improve the performance of their batteries. This is a huge step towards further commercialization of tin-based sodium-ion batteries, alongside existing companies in this space Unigrid Battery, Nanode, and Faradion.
Future outlook
Tin anodes for sodium-ion batteries are a rapidly emerging area of research, with significant interest from both academia and industry. Many established SIB companies are exploring the potential of tin-based anodes, despite challenges such as volume expansion.
However, ongoing advancements are addressing these issues, with promising solutions on the horizon. Companies like Nanode and Unigrid are making strides toward commercialising tin-based SIBs. Established battery manufacturers are evaluating tin solutions.
Moving forward, a collaborative dialogue is essential to accelerate development, and Tin Valley provides an ideal platform for driving these discussions and fostering innovation in this field.
References
- https://doi.org/10.1021/acsami.3c06868
- https://doi.org/10.1016/j.jallcom.2023.172968
- https://doi.org/10.1007/s10854-023-11893-7
- https://doi.org/10.1016/j.apsusc.2022.155859
- https://doi.org/10.1016/j.jallcom.2024.177048
- https://doi.org/10.1039/D3TA04905A
- https://doi.org/10.1021/acsami.4c13974
- https://doi.org/10.1016/j.est.2024.112407
- https://doi.org/10.1039/D4TC01332H
- https://doi.org/10.1016/j.jelechem.2024.118186
- https://doi.org/10.1007/s12598-023-02451-5
- https://doi.org/10.1016/j.electacta.2023.143401
- https://doi.org/10.1021/acsmaterialslett.3c00727
- https://doi.org/10.1002/aenm.202300351
- https://doi.org/10.1007/s12598-022-02174-z
- https://doi.org/10.1002/anie.202219177
- https://doi.org/10.1016/j.jpowsour.2023.232750
- https://doi.org/10.1039/d4nj02152e
- https://doi.org/10.1016/j.mseb.2025.118056
- https://doi.org/10.1016/j.electacta.2025.145730
- https://doi.org/10.1039/d4ta08119f
- https://doi.org/10.1039/d4nj04827j
- https://doi.org/10.1016/j.colsurfa.2025.136158
- https://doi.org/10.1016/j.jallcom.2025.178541
- https://doi.org/10.1002/celc.202400582
- https://doi.org/10.1016/j.jechem.2024.12.002
- https://doi.org/10.1021/acsmaterialslett.4c02033
- https://doi.org/10.1039/d4nj04756g
- https://doi.org/10.1016/j.ensm.2024.103906
- https://doi.org/10.1016/j.ensm.2024.103880
- https://doi.org/10.1007/s12598-024-03014-y
- https://doi.org/10.1016/j.jcis.2024.10.143
- https://doi.org/10.1002/advs.202408450
- https://doi.org/10.1039/d4qi01941e
- https://doi.org/10.1021/acsaem.4c01727
- https://doi.org/10.1063/5.0226473
- https://doi.org/10.1039/d4dt02094d
- https://doi.org/10.12422/j.issn.1006-396X.2024.04.010
- https://doi.org/10.1016/j.ces.2024.120670
- https://doi.org/10.1016/j.jssc.2024.124966
- https://doi.org/10.1002/smll.202405322
- https://doi.org/10.1002/smll.202405262
- https://doi.org/10.1016/j.cej.2024.154494
- https://doi.org/10.1021/acs.energyfuels.4c01895
- https://doi.org/10.1039/D4NR02418D
- https://doi.org/10.1149/1945-7111/acc895
- https://doi.org/10.1016/j.electacta.2024.144347
- https://doi.org/10.1016/j.jallcom.2024.174447
- https://doi.org/10.1016/j.diamond.2023.109903
- https://doi.org/10.1039/D4NJ00677A
- https://doi.org/10.1016/j.jcis.2024.07.070
- https://doi.org/10.1016/j.apsusc.2022.155859
- https://doi.org/10.1016/j.jpowsour.2022.232333
- https://doi.org/10.1016/j.electacta.2022.141590
- https://doi.org/10.1016/j.cej.2022.141243
- https://doi.org/10.1016/j.apsusc.2022.155672
- https://doi.org/10.1016/j.cej.2023.141629
- https://doi.org/10.1002/bte2.20220046
- https://doi.org/10.1016/j.apsusc.2022.155992
- https://doi.org/10.1016/j.cej.2023.141827
- https://doi.org/10.1002/eem2.12431
- https://doi.org/10.1016/j.cej.2023.142289
- https://doi.org/10.1016/j.cej.2023.142934
- https://doi.org/10.1016/j.jcis.2023.03.207
- https://doi.org/10.1016/j.est.2023.107354
- https://doi.org/10.1016/j.apsusc.2023.157938
- https://doi.org/10.1016/j.jallcom.2023.171562
- https://doi.org/10.1016/j.jallcom.2023.171809
- https://doi.org/10.1002/aenm.202302901
- https://doi.org/10.1016/j.jcis.2023.09.044
- https://doi.org/10.1016/j.jcis.2023.09.065
- https://doi.org/10.1016/j.ces.2023.119390
- https://doi.org/10.1016/j.compositesb.2023.111109
- https://doi.org/10.1007/s12598-023-02465-z
- https://doi.org/10.1021/acsami.3c18941
- https://doi.org/10.1016/j.jcis.2024.02.161
- https://doi.org/10.1016/j.jallcom.2024.174266
- https://doi.org/10.1002/smll.202311196
- https://doi.org/10.1016/j.electacta.2025.145833